| Standard No. |
Title |
Price(USD) |
Delivery |
Status |
Add To Cart |
| YY/T 1464-2016 |
Sterilization of medical devices—Requirements for the development,validation and routine control of a low temperature steam and formaldehyde sterilization process for medical devices |
 (USD) (USD) |
via email in
1-5 business day
|
VALID
|
|
| GB/T 18279.2-2015 |
Sterilization of health care products—Part 2:Guidance on the application of GB 18279.1 |
 (USD) (USD) |
via email in
1-5 business day
|
VALID
|
|
| YY/T 1302.2-2015 |
Physical requirements and microbiological performance of ethylene oxide sterilization—Part 2:Microbiological aspects |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 0567.5-2011 |
Aseptic processing of health care products—Part 5:Sterilization in place |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 1402-2016 |
Test method to demonstrate the suitability of the process challenge device(PCD) during steam sterilization |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| GB 18282.3-2009 |
Sterilization of health care products—Chemical indicators—Part 3:Class 2 indicator systems for use in the Bowie and Dick-type steam penetration test |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 1302.1-2015 |
Physical requirements and microbiological performance of ethylene oxide sterilization—Part 1: Physical aspects |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| GB/T 18280.3-2015 |
Sterilization of health care products—Radiation—Part 3:Guidance on dosimetric aspects |
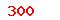 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 1265-2015 |
Evaluation of materials of medical device subject to moist heat sterilization |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| GB/T 19974-2018 |
Sterilization of health care products—General requirement for characterization of a sterilization agent and the development, validation and routine control of a sterilization process for medical devices |
 (USD) (USD) |
via email in
1-5 business day
|
VALID
|
|
| GB 18281.2-2000 |
Sterilization of health care products--Biological indicators--Part 2:Biological indicators for ethylene oxide sterilization |
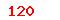 (USD) (USD) |
via email in
1-3 business day
|
ABOLISHED
|
|
| GB 18281.4-2015 |
Sterilization of health care products—Biological indicators—Part 4:Biological indicators for dry heat sterilization processes |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 0923-2014 |
Needleless access ports for fluid lines and blood lines—Test method for microbial ingress |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 0567.2-2005 |
Aseptic processing of health care products -- Part 2: Filtration |
|
via email in
1-3 business day
|
VALID
|
please email coc@codeofchina.com for quotation
|
| GB/T 19973.1-2015 |
Sterilization of medical devices—Microbiological methods—Part 1:Determination of a population of microorganisms on products |
 (USD) (USD) |
via email in
1-5 business day
|
VALID
|
|
| GB/T 24628-2009 |
Sterilization of health care products—Biological and chemical indicators—Test equipment |
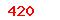 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 0567.3-2011 |
Aseptic processing of health care products—Part 3:Lyophilization |
|
via email in
|
VALID
|
please email coc@codeofchina.com for quotation
|
| GB 18279.1-2015 |
Sterilization of health care products—Ethylene oxide—Part 1:Requirements for development,validation and routine control of a sterilization process for medical devices |
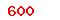 (USD) (USD) |
via email in
1-5 business day
|
VALID
|
|
| YY/T 0615.1-2007 |
Requirements for medical devices to be designated STERILE—Part 1:Requirements for terminally sterilized medical devices |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| GB 18282.5-2015 |
Sterilization of health care products—Chemical indicators—Part 5:Class 2 indicators for Bowie and Dick-type air removal tests |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
|
|

 (USD)
(USD)  (USD)
(USD) 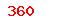 (USD)
(USD)  (USD)
(USD) 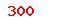 (USD)
(USD) 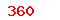 (USD)
(USD) 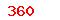 (USD)
(USD) 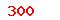 (USD)
(USD)  (USD)
(USD)  (USD)
(USD) 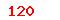 (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD) 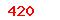 (USD)
(USD) 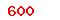 (USD)
(USD)  (USD)
(USD)  (USD)
(USD)