| Standard No. |
Title |
Price(USD) |
Delivery |
Status |
Add To Cart |
| GB 12263-2005 |
Artifical heart-lung machine—Water heating/cooling system |
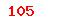 (USD) (USD) |
via email in
1-3 business day
|
ABOLISHED
|
|
| YY/T 0987.1-2016 |
Implants for surgery—Magnetic resonance compatibility—Part 1:Safety marking |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 1647-2019 |
Joint replacement implants—Shoulder prostheses—Standard test method for static evaluation of glenoid locking mechanism in shear |
 (USD) (USD) |
via email in
1-3 business day
|
TO BE VALID
|
|
| GB/T 19701.2-2016 |
Implants for surgery—Ultra-high-molecular-weight polyethylene—Part 2:Moulded forms |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 0956-2014 |
Implant for surgury—Staples with parallel legs for orthopaedic use—General requirements |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| GB/T 30659-2014 |
External limb prostheses and external orthoses—Requirements and test methods |
 (USD) (USD) |
via email in
1-8 business day
|
VALID
|
|
| GB/T 13461-2008 |
Modular below knee prosthesis |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 0597-2006 |
Clip Applicator |
|
via email in
1-3 business day
|
VALID
|
please email coc@codeofchina.com for quotation
|
| GB/T 13461-1992 |
Synthetic resin blow knee prostheses |
|
via email in
1-3 business day
|
ABOLISHED
|
please email coc@codeofchina.com for quotation
|
| GB/T 31181-2014 |
Prosthetics—Testing of ankle-foot devices and foot units—Requirements and test methods |
 (USD) (USD) |
via email in
1-8 business day
|
VALID
|
|
| YY/T 0726-2009 |
Instrumentation for use in association with non-active surgical implants—General requirements |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY 0459-2003 |
Implants for surgery—Acrylic resin cements |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| GB 16174.2-2015 |
Implants for surgery—Active implantable medical devices—Part 2:Cardiac pacemakers |
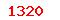 (USD) (USD) |
via email in
1-10 business day
|
VALID
|
|
| GB 16174.1-2015 |
Implants for surgery. Active implantable medical devices. Part 1:General requirements for safety, marking and for information to be provided by the manufacturer |
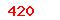 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| GB 12279-1990 |
Cardiac valve prostheses general technical specification |
|
via email in
1-3 business day
|
ABOLISHED
|
please email coc@codeofchina.com for quotation
|
| GB 12279-2008 |
Cardiovasular implants - Cardiac valve prostheses |
 (USD) (USD) |
via email in
1-5 business day
|
VALID
|
|
| GB/T 13810-1997 |
Wrought titanium and titanium alloy for surgical implants |
|
via email in
1-3 business day
|
ABOLISHED
|
please email coc@codeofchina.com for quotation
|
| GB 14722-1993 |
Endoskeletal above knee prosthesis |
|
via email in
1-3 business day
|
ABOLISHED
|
please email coc@codeofchina.com for quotation
|
| YY/T 1576-2017 |
Tissue engineering medical device products—Standard practice for implantation assessment of absorbable/resorbable biomaterials |
 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
| YY/T 0962-2014 |
Cross-linked sodium hyaluronate gel for plastic surgery |
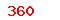 (USD) (USD) |
via email in
1-3 business day
|
VALID
|
|
|
|

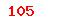 (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD)  (USD)
(USD) 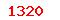 (USD)
(USD) 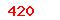 (USD)
(USD)  (USD)
(USD)  (USD)
(USD) 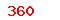 (USD)
(USD)